COVID-19 Generates Hyaluronan Fragments that Directly Induce Endothelial Barrier Dysfunction
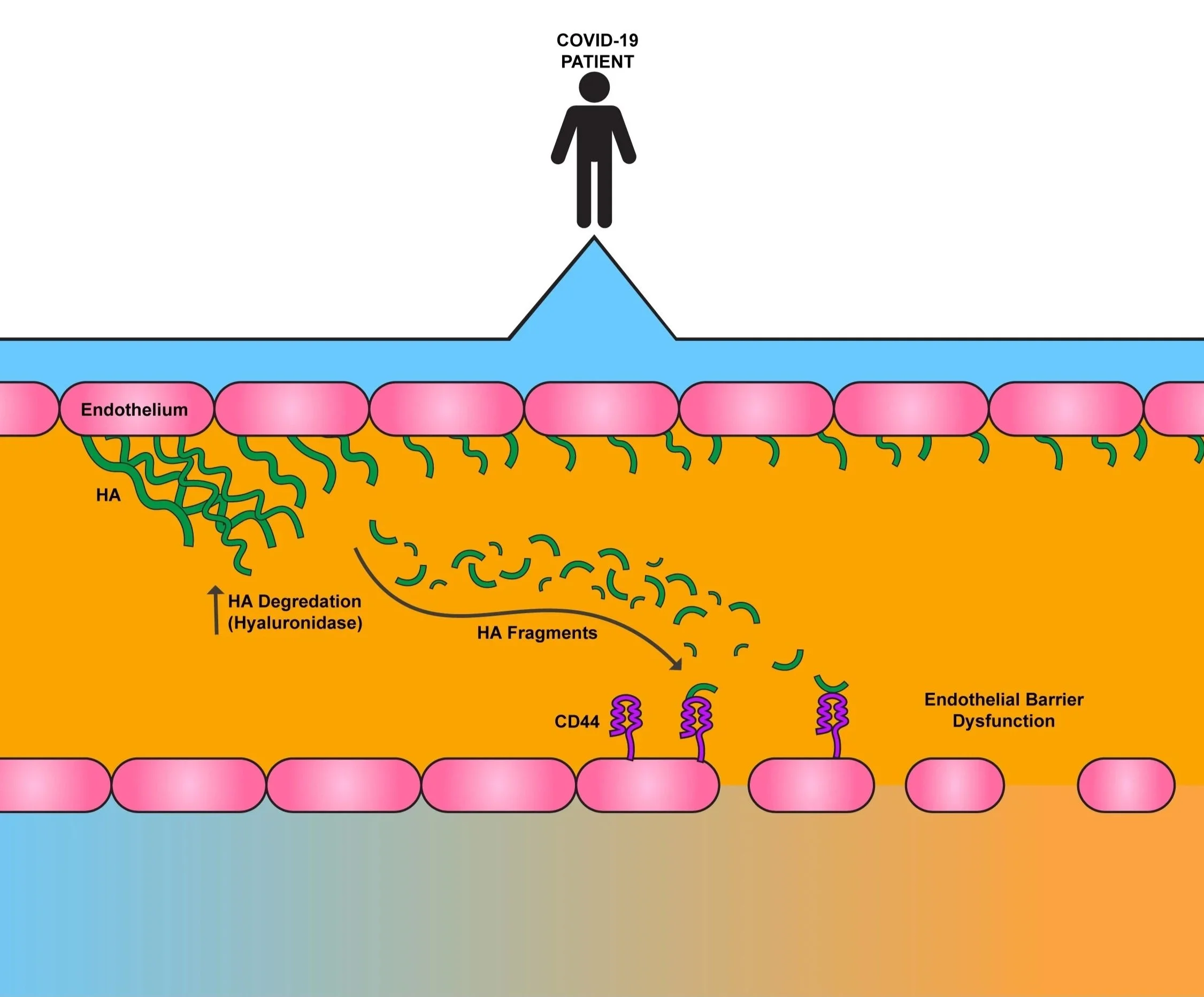
COVID-19 disrupts the function of the cells outlining the circulatory system, or the endothelium. While this includes vascular leakage and release of pro-inflammatory molecules into the blood, it also includes cleaving of Hyaluronan (HA) within the extracellular matrix (ECM), sending HA fragments into circulation. What these fragments of HA could do during COVID-19 had yet to be discovered.
First, we measured how much HA, heparin sulfate, and chondroitin sulfate were floating around in the bloodstreams of patients. If the ECM was being degraded during COVID-19, we should see increased levels of fragments of all three ECM components. Indeed, this was exactly what we found.
What was cause of this increase, though? When we checked the levels of the enzymes responsible for digesting these ECM components, all three were significantly higher in COVID-19 patient blood compared to healthy individuals. We decided to continue examining HA concentrations in patients. We found that as HA fragmentation increased in COVID-19 patients, the worse their disease presented. We then took plasma from COVID patients and healthy patients and use it to coat endothelial cells. The COVID samples led to not only a stark increase in HA digestion, but they also increased the cell’s production of HA. Therefore, patient cells are making and breaking their HA at an alarming rate.
We examined the HA fragments circulating within the plasma further, specifically determining their size. Generally, smaller HA fragments in the blood stream are considered “pro-inflammatory,” meaning that they can drive the immune system to respond to a given threat. In healthy individuals, the HA fragments circulating in the blood are quite large, over 100 kDa. However, most of the HA fragments that we identified in COVID-19 patients were less than half that size, which is the range considered for the “pro-inflammatory” fragment form.
This led us to wonder about what these fragments were doing to endothelial cells. Based on previously published literature, we decided to test whether COVID-derived HA fragments disrupted the cellular barriers within a patient’s body. When we incubated endothelial cells with COVID-19 HA fragments, we found that the presence of HA fragments from COVID patients make the cells more “leaky.” Increased leaking can lead to swelling in a patient. When we inhibited the activity of the molecule ROCK, known for maintaining cells shape (and therefore barrier integrity), we found that the HA fragments did not induce nearly as much barrier leakage. Therefore, the fragments are altering that specific pathway. To determine what HA receptor the fragments were binding to on the cells, we effectively deleted multiple receptors in our cells and then incubated with the fragments. Through this experiment, we determined that the HA fragments bind to the receptor CD44.
Therefore, we can conclude that during COVID-19, production and digestion of HA occurs, making a high amount of small HA fragments. We can deduce that with more of the “pro-inflammatory” fragments circulating in a patient’s blood, the more the patient’s cellular barriers leak, leading to worse disease symptoms.