Thrombin Cleavage of Inter-a-inhibitor Heavy Chain 1 Regulates Leukocyte Binding to an Inflammatory Hyaluronan Matrix
Lay Summary
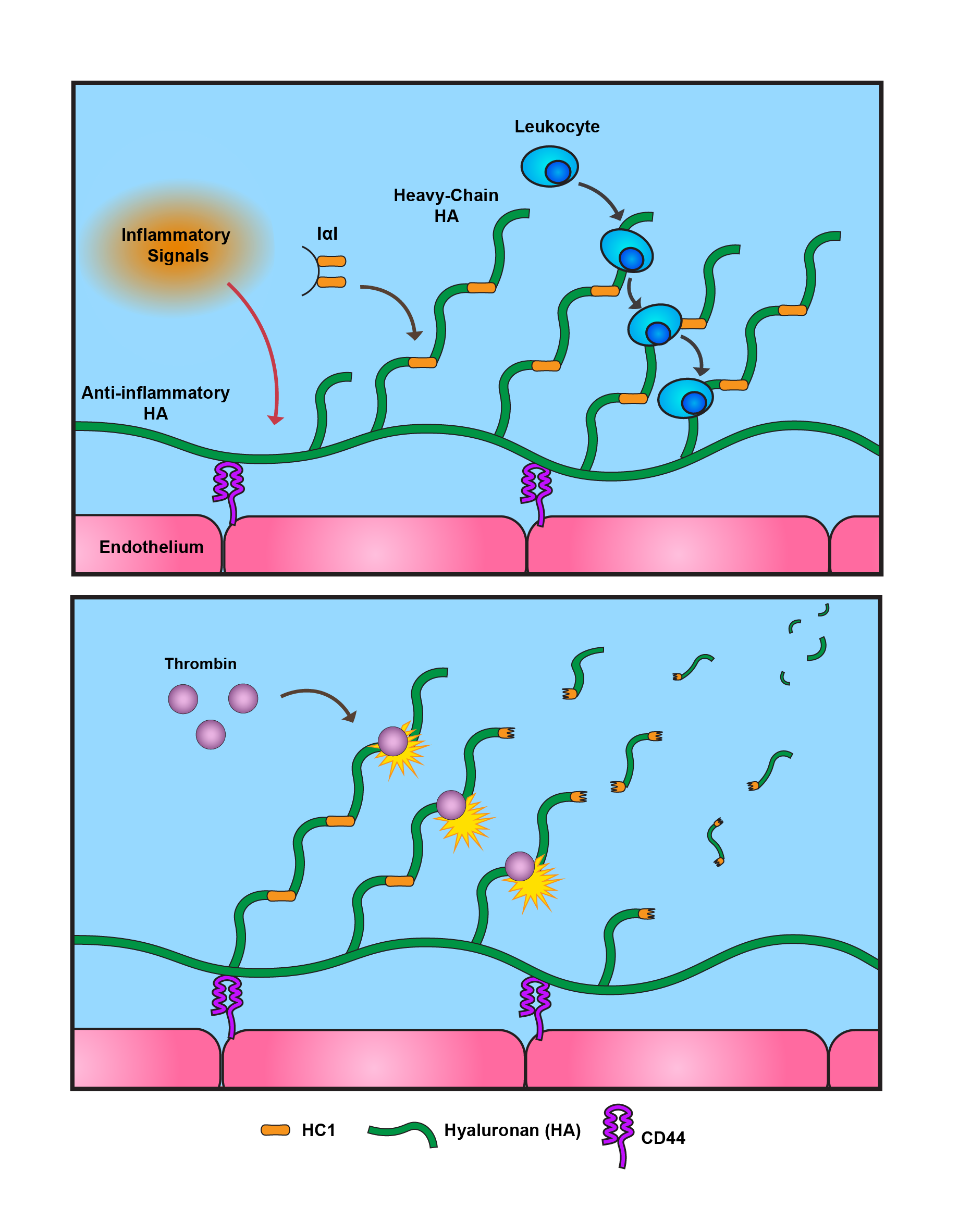
During Inflammatory Bowel Disease (IBD), cells produce the pro-inflammatory form of hyaluronan (HA) that acts as binding sites for incoming immune cells. This pro-inflammatory form is called “heavy-chain HA,” so named for the additional heavy chains deposited onto the HA that allow the HA to branch out into the extracellular environment.
Thrombin is a protease, meaning that its primary job is to cut up other proteins. Its concentration rises significantly in IBD patient blood, lending thrombin to become a protease of interest when it comes to a patient’s disease state. Thrombin is well studied in the clotting pathway, but up until this research, whether thrombin played a role in maintaining heavy-chain HA had not been explored.
In this paper, we started by chemically stimulating intestinal smooth muscles cells, encouraging them to make their own heavy-chain HA. To our surprise, when we then incubated with thrombin, immune cells lost the ability to bind effectively to the cells. This wasn’t due to thrombin blocking the immune cells from binding, nor to thrombin activating these cells. When we examined the heavy-chain HA under a microscope, we found that the presence of thrombin led to the reduction of the heavy-chain HA. These results led us to ask: what in the pro-inflammatory HA structure is thrombin cleaving?
First, we checked to see if any components of heavy-chain HA could be a target for thrombin. We found a site on Heavy Chain 1 (HC1) where thrombin could cut, leading us to speculate that this was what thrombin was targeting. Sure enough, we found a site on HC1 where thrombin could cleave. And indeed, combining thrombin with HC1 led to clear fragmentation of HC1. We confidently concluded that thrombin targets HC1 in our heavy-chain HA, leading to less leukocytes binding to cell surfaces due to the loss of the HA structures.